ESG / CSR
Industries
Ocean Acidification: Causes, Issues and Solutions



We often talk about the impact of rising carbon dioxide emissions on our climate, but not many people realize that it's also changing the chemistry of our oceans.
Ocean acidification may not make as many headlines as climate change, but it poses a serious and growing threat to marine life and to the ecosystems and economies that depend on it. Scientists now recognise it as one of the most urgent environmental issues of our time – one that threatens the health of the global ocean and everything that depends on it.
In this article, we’ll break down what ocean acidification is, what’s causing it, and what we can do to protect the ocean and its incredible biodiversity.
What is ocean acidification?
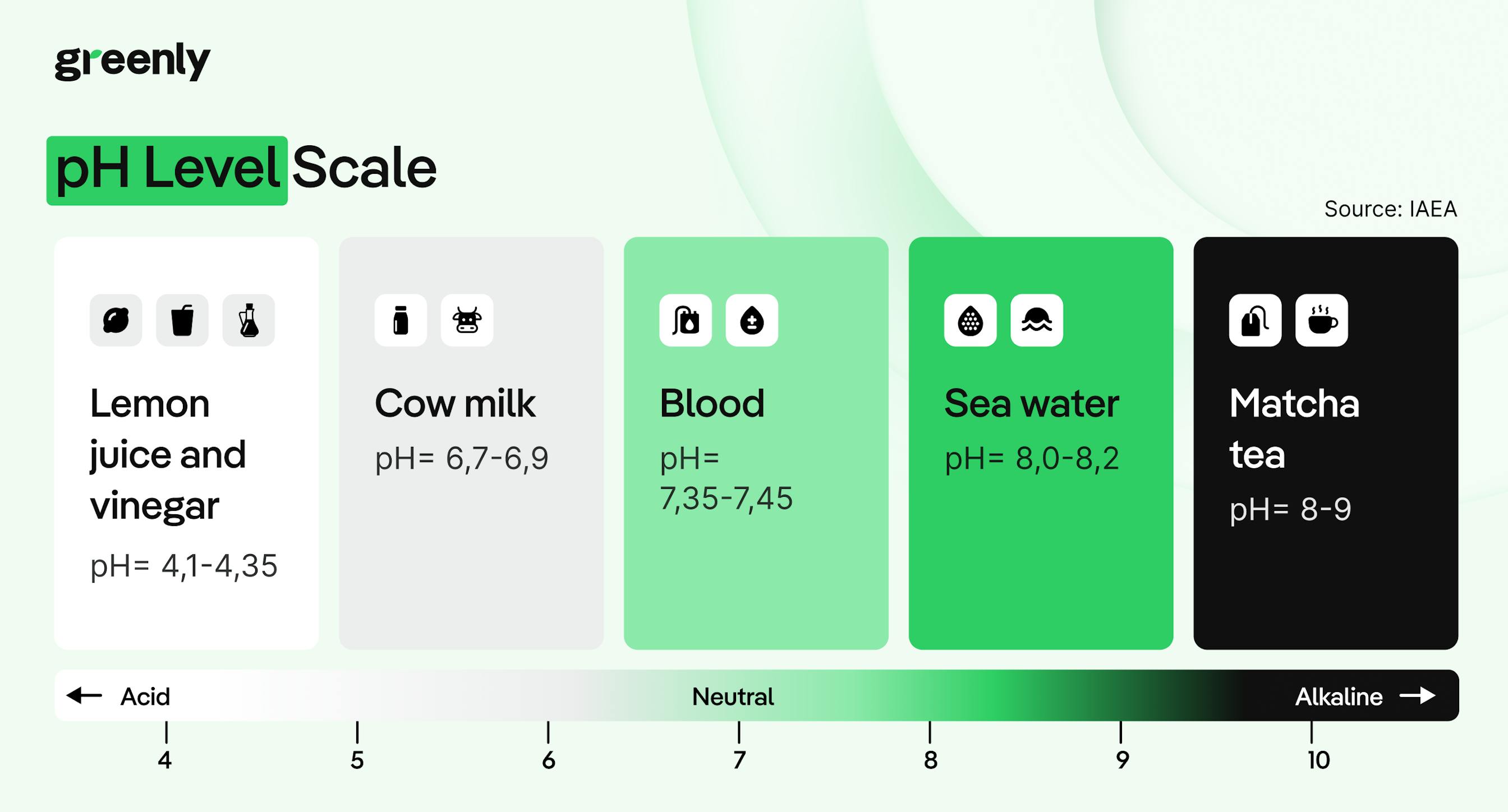
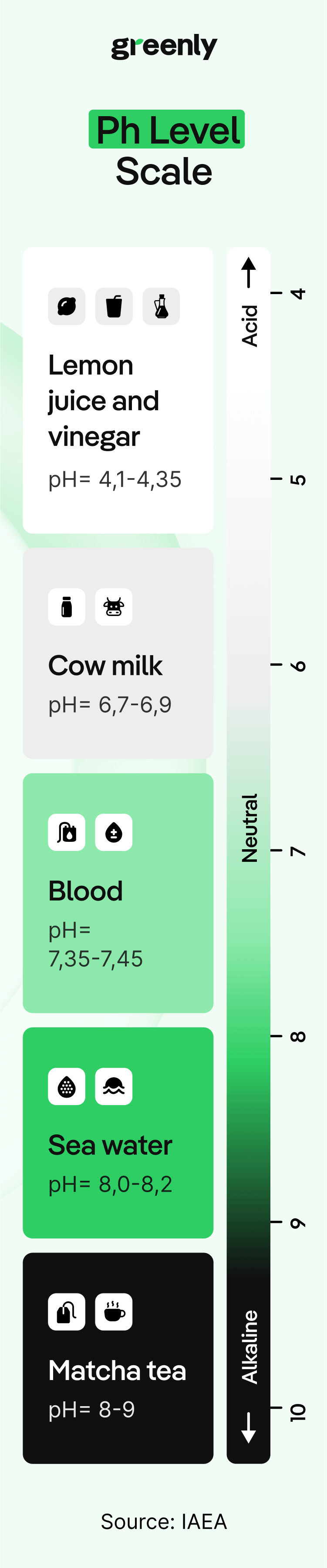
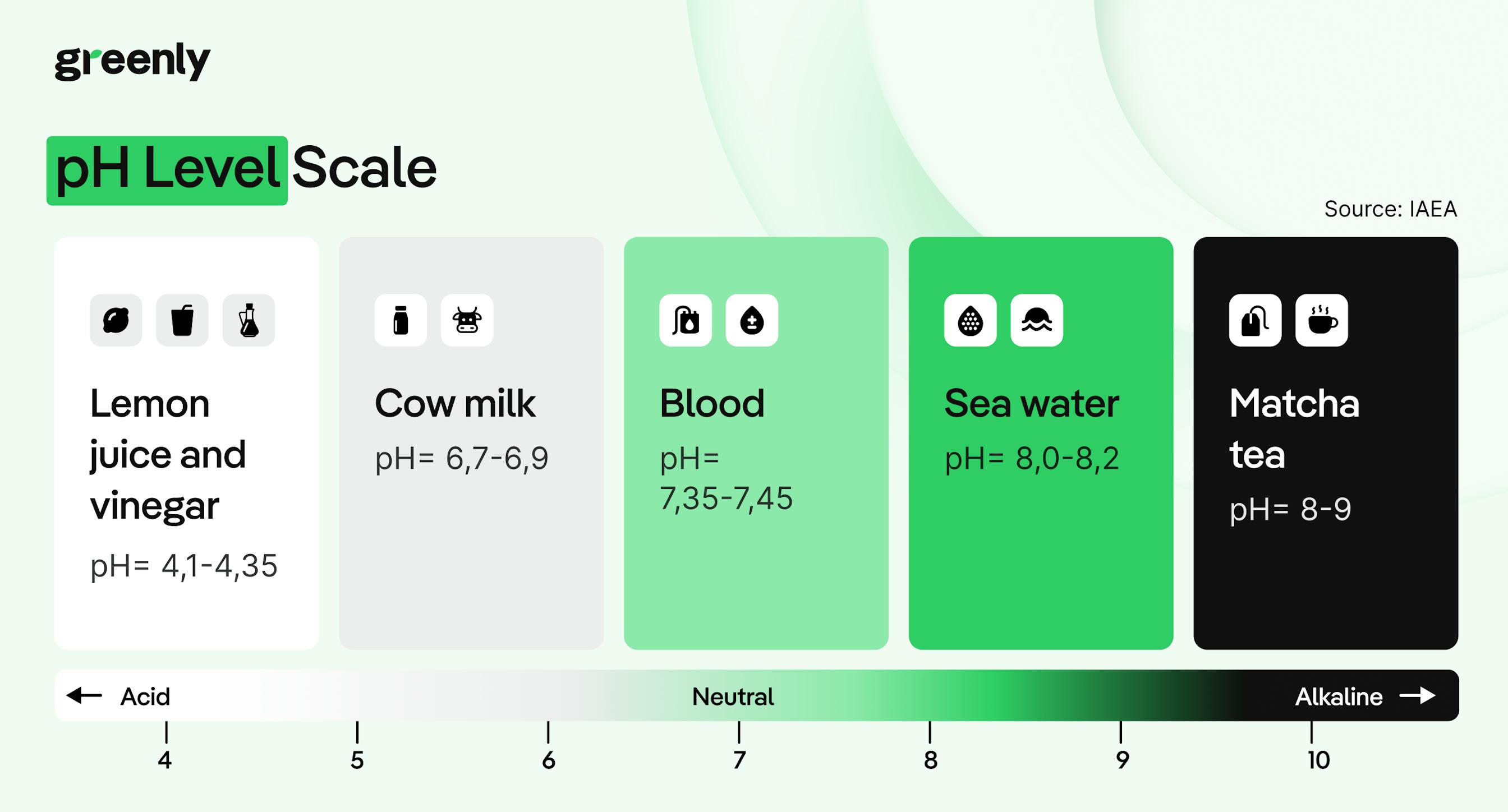
When carbon dioxide dissolves in seawater, it triggers a series of chemical reactions that make the water more acidic. This might not sound like a big deal, but it affects the delicate balance of marine ecosystems. The drop in pH reduces the availability of carbonate ions, which many marine species - including corals, shellfish, and some types of plankton - need to build their shells and skeletons.
While the effects of climate change on land are often visible (rising temperatures, wildfires, drought), ocean acidification is harder to see, but no less serious. It’s quietly reshaping the chemistry of our oceans, with consequences that ripple through the entire marine food web.
Why is ocean acidification happening?
Ocean acidification is the result of rising levels of carbon dioxide (CO2) in the Earth's atmosphere, much of it caused by human activity.
The ocean plays a particularly important role. It is able to absorb carbon dioxide in two main ways:
Through physical processes: where CO₂ dissolves into surface waters, especially in colder waters.
Through biological processes: where microscopic plants like phytoplankton absorb CO₂ during photosynthesis, helping to transfer it into the deep ocean.
But over the last two centuries, that balance has been lost.
Since the Industrial Revolution, human activities like burning fossil fuels, clearing forests, changing land use, and producing cement have released more carbon dioxide into the atmosphere than ever before – far more than the natural system can absorb. As a result, the ocean has taken in hundreds of billions of tonnes of excess CO2, pushing its chemistry beyond natural limits.
In other words, the ocean has been buffering the impact of climate change for decades, but now, we’re seeing the cost of that hidden service. And unless emissions fall, the trend will only continue.
What happens when oceans absorb more CO2?
When carbon dioxide dissolves in seawater, it doesn’t just sit there, it triggers a chain of chemical reactions that alter the water’s composition.
But there’s a second consequence: those extra hydrogen ions also react with carbonate ions (CO₃²⁻), reducing their availability in seawater. That matters because many marine organisms, from tiny plankton to corals and shellfish, rely on carbonate ions to build their shells and skeletons. As acidity rises and carbonates become scarce, these species struggle to survive and grow. Some species may even experience shell dissolution, meaning their existing shells can literally start to erode in more acidic waters.
This shift in ocean acidity is already affecting sensitive ecosystems, and it’s happening faster than many marine species can adapt.
Ocean acidification effects
The consequences of ocean acidification are far-reaching, affecting everything from coral reefs and shellfish to fish behaviour and the balance of entire ecosystems. Below, we explore the key impacts across different parts of the marine environment.
Corals are especially vulnerable to ocean acidification. As marine calcifiers, they build their skeletons from calcium carbonate, and when carbonate levels drop, corals grow more slowly and their structures become weaker and more prone to erosion. In severe cases, acidification can even start to dissolve existing coral. Combined with ocean warming, these stressors threaten the survival of coral reefs worldwide.
Some coral species are showing resilience by adapting to use bicarbonate instead of carbonate ions, or by temporarily surviving without a skeleton. But this doesn’t mean coral reefs are safe – we’re likely to see major shifts in reef composition and biodiversity in the decades ahead.
Since coral reefs support around 25% of all marine life, any change in their structure affects a huge range of species that depend on them for food, habitat, and protection.
Like corals, many calcifying organisms, including mussels, clams, oysters, and sea urchins, depend on carbonate ions to form their calcium carbonate shells. As acidity increases and carbonate ion concentrations become scarce, their shells form more slowly and are structurally weaker.
Research shows that by the end of the century, mussel shells could be 25% thinner, and oyster shells 10% thinner, leaving them more vulnerable to predators and environmental stress.
Ocean acidification doesn’t just affect the shells themselves. For example, the fibres mussels use to cling to rocks become less effective, and oyster larvae can struggle to form their first shells, which they need to begin feeding and growing. This has already caused major disruptions in commercial oyster farms, particularly in the Pacific Northwest, where some hatcheries have lost up to 75% of their oyster larvae.
Plankton may be microscopic, but they’re the foundation of the marine food chain. Some plankton species, like coccolithophores and certain types of zooplankton, use calcium carbonate to form protective shells. These shells are highly sensitive to changes in ocean pH and can begin to dissolve under more acidic conditions.
Zooplankton are a primary food source for many fish and marine mammals, while phytoplankton not only feed zooplankton but also play a vital role in absorbing CO₂ from the atmosphere through photosynthesis, similar to trees on land.
Research shows that different phytoplankton species react differently to acidification: some may decline or migrate, while others may thrive. This imbalance has the potential to reshape entire ecosystems.
Fish don’t rely on shells, but that doesn’t mean they’re unaffected. Acidifying waters can lead to acidosis, a condition where the pH of a fish’s blood drops due to the uptake of carbonic acid. This can alter their metabolism, growth, and even behaviour.
For example, studies have shown that in more acidic waters:
- Clownfish struggle to detect predators or navigate back to their home reef
- Juvenile fish exhibit riskier behavior and are less responsive to danger
Over time, this could reduce survival rates and disrupt reproductive success, especially for species already under pressure from overfishing or habitat loss.
Not all species are harmed by ocean acidification. Algae, which use CO₂ for photosynthesis, may actually thrive in more acidic conditions. But more algae isn’t always a good thing.
Excess nutrients and higher CO₂ levels can trigger harmful algae blooms, where fast-growing algae release toxins that are dangerous to marine life, and even humans. These blooms can:
- Suffocate fish and shellfish
- Contaminate seafood
- Cause respiratory and gastrointestinal illness in people
Depending on the species, some algal blooms can be life-threatening or cause widespread ecosystem disruption.
The impact of ocean acidification ripples far beyond individual species. When foundational species like corals, shellfish, and plankton are weakened or lost, the entire marine food web becomes unstable.
Predators lose access to prey, species migrate in search of new habitats, and ecosystem services, like carbon storage, oxygen production, and food supply, begin to break down.

The economic impacts of ocean acidification
Ocean acidification also comes with a heavy economic cost, particularly for communities that rely on healthy oceans for income and food security.
Tracking the damage so far
We're no longer just predicting the effects of ocean acidification, we're now seeing them unfold in real time. From dissolving shells to collapsing coral structures, marine life is already under pressure. But the problem doesn’t affect all parts of the world equally.
The rate and severity of ocean acidification can vary significantly between regions, depending on local conditions like water temperature, circulation, and freshwater input. Understanding these regional differences is key to developing strategies that work, both for protecting ecosystems and supporting the communities that rely on them.
The Arctic is one of the fastest-changing regions on Earth, and it’s also where ocean acidification is happening most rapidly. Several factors make Arctic waters especially vulnerable:
- Cold water absorbs more CO₂, which accelerates acidification compared to warmer regions.
- Melting sea ice reduces the ocean’s ability to reflect sunlight, warming the water and compounding chemical changes.
- Freshwater from melting glaciers dilutes the ocean and weakens its natural ability to buffer changes in seawater pH.
These shifts pose serious risks to Arctic marine life, particularly plankton and shellfish, which are the foundation of the local food web.
What happens in the Arctic also has wider implications, influencing ocean circulation and climate systems across the globe.
The Coral Triangle, which spans parts of Indonesia, Malaysia, the Philippines, and Papua New Guinea, is one of the most biologically rich marine areas in the world. But its biodiversity doesn’t make it immune to acidification; in fact, it may make the region even more vulnerable.
- With so many coral species, acidification is likely to hit unevenly – some may adapt, others may disappear, altering reef composition.
- The region also faces other stressors, like overfishing, pollution, and coastal development, all of which weaken the resilience of marine ecosystems.
The combined effect? Coral reefs in the Coral Triangle could lose both structure and biodiversity – a dangerous outcome for the thousands of species and millions of people who depend on them.
Regions like the North Atlantic Ocean and the North Pacific Ocean, off the coasts of Europe and North America, are also experiencing acidification, but in more varied ways. Here, local factors like water temperature, upwelling, and ocean currents shape how fast and how severely acidification takes hold.
- pH changes can vary widely from one stretch of coastline to another.
- These waters support large commercial fisheries and shellfish farms, making them economically sensitive to even small changes in water chemistry.
In the Pacific Northwest, for example, oyster hatcheries have already seen major losses due to acidification, affecting food supply chains and putting livelihoods at risk.
Tropical regions, including the Caribbean and Pacific Islands, are also on the frontlines of ocean acidification.
- Coral reefs, already stressed by warming waters, are highly sensitive to even slight drops in pH, which weaken their skeletons and slow their growth.
- Complex ocean circulation patterns can change how acidification appears in different parts of the tropics, influencing which areas are hit hardest.
Since coral reefs underpin tourism, fisheries, and coastal protection in these regions, their decline has wide-reaching consequences – not just for marine life but for entire economies.
The outlook: what lies ahead?
As carbon dioxide levels continue to rise, so too will the acidity of our oceans. And unless global emissions are drastically reduced, the situation will worsen fast.
Ocean acidification is not a distant threat, it’s happening now. The longer we delay action, the harder it will be to protect the biodiversity, food systems, and economies that depend on a stable ocean.
What can we do about ocean acidification?
Ocean acidification may be a global challenge, but it’s not without solutions. While the chemistry of our oceans is changing, we still have time to slow or even reverse some of its effects. That starts with tackling the root cause: excess carbon dioxide.
Here are the most important areas of action:
Drastically reduce carbon emissions
The most direct and urgent solution to ocean acidification is to cut greenhouse gas emissions, especially carbon dioxide. As long as we continue burning fossil fuels at current rates, the oceans will keep absorbing more carbon and becoming more acidic.
Reversing this trend means transitioning away from coal, oil, and gas. Every sector, from transport and energy to agriculture and manufacturing, needs to decarbonize fast. This shift requires bold, coordinated efforts from governments, businesses, and individuals alike.
Until global emissions begin to fall sharply, ocean acidification will continue to accelerate.
Restore and protect natural carbon sinks
Even if we stopped emitting carbon tomorrow, the oceans would still be absorbing excess CO2 already present in the atmosphere. That’s why we also need to restore marine ecosystems and strengthen the planet’s natural carbon sinks – systems that play a vital role in regulating natural processes like carbon cycling and climate balance.
This includes:
Reforestation and afforestation: Planting trees in deforested or previously unforested areas helps absorb CO₂ from the atmosphere and rebuilds natural carbon sinks.
Sustainable agriculture and land use: Improving farming practices and land management can enhance carbon retention in soils while supporting biodiversity and food production.
Soil carbon enhancement: Techniques like cover cropping, composting, and reduced tillage increase the amount of organic matter in soil, boosting its carbon storage capacity.
Protection of blue carbon ecosystems: Safeguarding coastal habitats such as mangroves, seagrasses, and salt marshes preserves vital carbon sinks and strengthens shoreline resilience.
We can also support emerging technological carbon removal methods, such as direct air capture, enhanced weathering, and ocean alkalinity enhancement, though these are still in early stages of deployment.
Strengthen policy and international cooperation
Ocean acidification is a global problem, and like all global problems, it requires coordinated action. While the science has become increasingly clear, policy frameworks have been slower to catch up. A number of international agreements and national strategies now include measures to address ocean acidification, but most remain limited in scope, and implementation is still uneven.
International and national frameworks
Several existing frameworks help tackle ocean acidification by targeting its root cause (CO2 emissions), supporting scientific research, or encouraging the protection of vulnerable marine ecosystems.
| Initiative | How it helps |
|---|---|
|
Paris Agreement (2015)
|
Cuts CO₂ emissions — the most direct way to slow acidification. |
|
UN Decade of Ocean Science (2021–2030)
|
Promotes international collaboration, research, and data sharing on ocean chemistry. |
|
Convention on Biological Diversity (CBD)
|
Encourages countries to integrate acidification into biodiversity planning. |
|
BBNJ Agreement (2023)
|
Expands ocean governance into areas beyond national jurisdiction, including protections for vulnerable ecosystems affected by acidification. |
|
Ocean Acidification Research for Sustainability (OARS)
|
Strengthens scientific cooperation and aims to connect research with policy at all levels. |
|
OA Alliance
|
Supports subnational and national governments in developing Ocean Acidification Action Plans. |
|
National policies (e.g. UK Marine Strategy, NOAA OA Program, EU Marine Strategy Directive)
|
Fund monitoring and research programmes, and in some cases, support regional adaptation planning. |
These efforts matter because policy drives change at scale. They help set emissions targets, fund research, standardise monitoring, and shape national strategies for protecting marine ecosystems.
Where are the gaps?
Despite growing momentum, current frameworks still fall short in several key areas:
What needs to change
To meaningfully address ocean acidification, we need to move from recognition to action. That means:
If policy is to live up to its potential, it must do more than monitor change, it needs to help shape a different future. Ocean acidification is not just a scientific issue; it’s a governance challenge.
The path forward
No single solution can fix ocean acidification on its own. But taken together, through emissions cuts, ecosystem restoration, and coordinated policy, we can begin to stabilize ocean chemistry and protect the marine life that billions of people depend on.
Public awareness, scientific research, and political will all have a role to play. The sooner we act, the better chance we have of ensuring the oceans remain a thriving, life-sustaining system for generations to come.
A moment for global action
This month, world leaders, scientists, and civil society are gathering in Nice, France, for the United Nations Ocean Conference (UNOC-3) - a major milestone for international ocean governance.
The summit comes at a critical time, as ocean acidification continues to accelerate. Among its key priorities are:
There’s growing recognition that ocean acidification must be treated as a core climate and biodiversity issue, not a side note. This summit is a chance for governments to commit to deeper emissions cuts, expand protections for carbon-rich marine ecosystems, and bring acidification into the heart of international climate policy.
What’s needed now is follow-through, not just new declarations, but real, coordinated action.
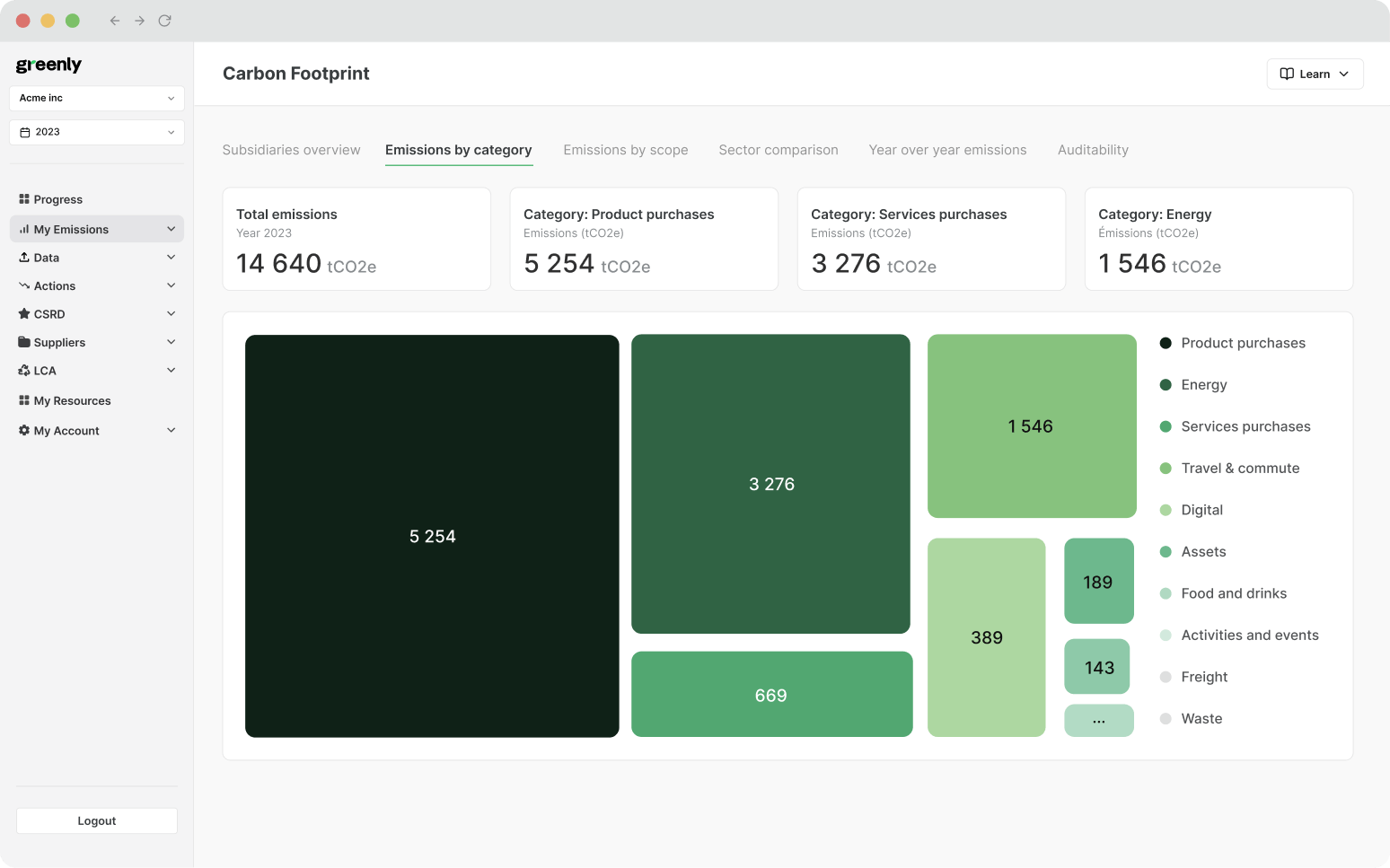
What about Greenly?
At Greenly, we help companies take control of their carbon emissions, from understanding where they come from to building effective strategies to reduce them.
Our carbon management services include:
- Comprehensive carbon footprinting across Scope 1, 2, and 3 emissions
- Customized emissions reduction plans tailored to your industry
- Supply chain visibility tools to help you work with lower-emission suppliers
- Support for climate disclosure and certification, including SBTi alignment
Whether you're just starting out or looking to lead your sector in sustainability, our platform and expert team make it easier to turn climate commitments into measurable progress. Get in touch with us today to find out more.